Simultaneous Approval of Two Cardiac MRI AI Analysis Products by Regulatory Authorities
Writer 최고관리자
Date 21-12-17 17:32
Views 1,124
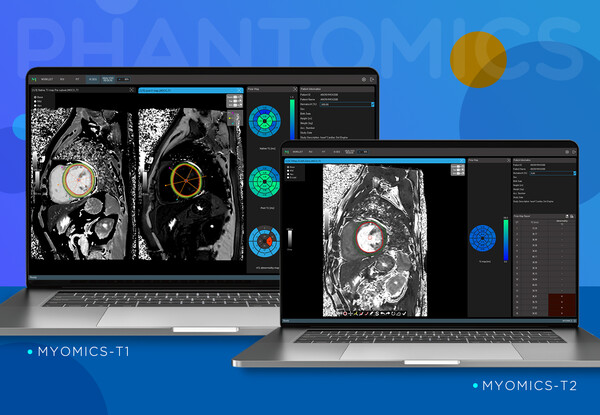
AI medical imaging diagnosis software company, Phantomics (CEO Kim Pan-ki, Choi Byung-wook) announced on the 17th that its automatic analysis AI diagnostic support software, "Myomics-T1" and "Myomics-T2," for cardiac magnetic resonance imaging (MRI) received simultaneous sales approval from the Ministry of Food and Drug Safety.
The approved Myomics-T1 and Myomics-T2 software are designed to automatically analyze relaxivity, a measure of how different tissues respond to the magnetic field in MRI, to identify areas with abnormalities in the heart muscle. The technology is characterized by enhancing readability in complex image readings. Relaxivity is a measurable image biomarker that reflects the molecular environment of substances and tissues in the body, as it is determined by the time it takes for hydrogen atoms in the body to absorb energy from electromagnetic waves and transfer the absorbed energy to the surrounding molecular environment (structure and size of molecules, etc.) in a strong magnetic field within MRI.
Recently, relaxivity has been used in the diagnosis of myocarditis, which causes inflammation in the heart muscle due to the COVID-19 pandemic, and in the diagnosis of image biomarkers such as amyloidosis, which involves abnormal protein accumulation in the heart muscle, and Fabry's disease, which involves fat accumulation. Unlike X-rays or CT scans, cardiac MRI does not involve any radiation exposure and can measure various quantitative indicators, including relaxivity, in living tissues. Clinical studies have shown that MRI can be used as an alternative diagnostic method to tissue examination by surgery, enabling convenient diagnosis of heart diseases without surgery. Traditional myocardial tissue examination involved the removal of a rice grain-sized heart muscle tissue sample through surgery, which had to be observed under a microscope to identify disease-induced tissue changes.
CEO Kim Pan-ki said, "Approval of the two Myomics products by the Ministry of Food and Drug Safety means that three products have been approved in Korea, and the Myomics-Q product has also recently been approved by the US FDA," adding that the company will continue to develop Myomics products to diagnose the world's leading cause of death, heart disease, more safely and accurately. He also stated that the company aims to provide diagnostic technologies that can overcome geriatric diseases in the coming super-aging society and selectively determine high-cost medical procedures.
Article: http://www.bosa.co.kr/news/articleView.html?idxno=2164694
