Phantomics received approval from the US FDA
Writer 최고관리자
Date 21-12-10 17:28
Views 934
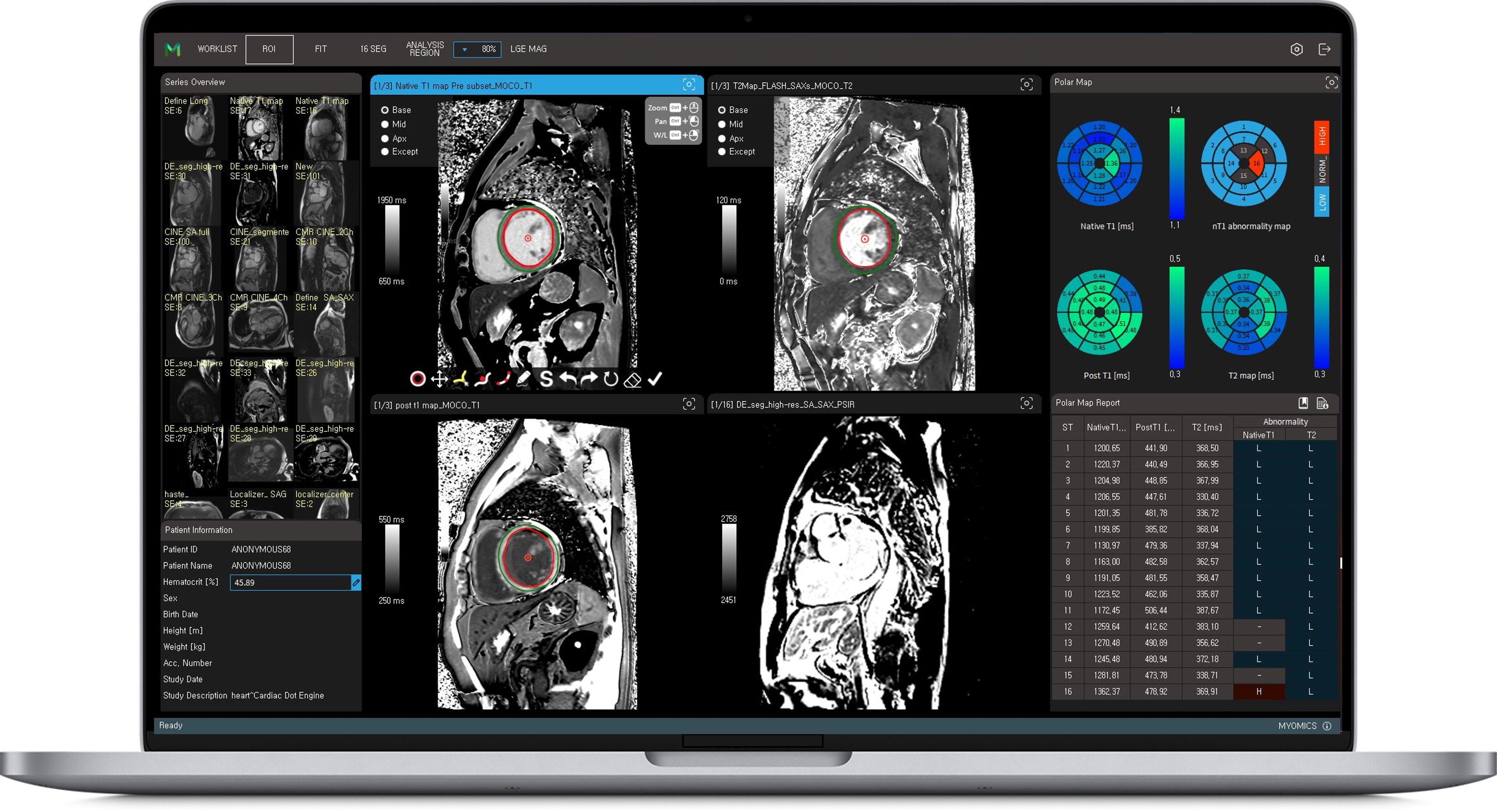
Phantomics, a next-generation AI imaging diagnosis startup, received approval from the US FDA on November 23, 2021, for its "Myomics-Q" software, which automatically analyzes cardiac MRI images.
In just two years since its establishment, Phantomics has secured both domestic medical device approval and US FDA approval. Phantomics' Myomics products are designed to assist medical professionals in diagnosing the leading cause of death worldwide based on quantitative and objective evidence obtained from imaging biomarkers in cardiac MRI images, backed by specialized knowledge of medical image physics and abundant clinical evidence.
The products can assist in diagnosing the disease based on evidence for the 523 million patients worldwide. The Myomics product line of Phantomics obtained approval from the Ministry of Food and Drug Safety in September 2020 and is expected to obtain two additional approvals by the end of this month. With this FDA approval, Phantomics can sell Myomics not only in Korea but also in the United States, which is the world's largest pharmaceutical and medical device market. Based on this approval, Phantomics plans to actively pursue global market sales.
Article: https://www.pharmnews.com/news/articleView.html?idxno=109635
